Table of Contents
Many investigators do not know the potential problems with antibody specificities, and the need for the careful documentation of antibody characterization for each antibody is used. This review will center on the principle of antibody and their definition of rules set for what information an investigator needs to obtain before considering the use of an antibody in a deep scientific investigation. Ever since Coons (1958), IHC described indirect immunohistochemical (IHC) staining, it has become a standard method employed in many laboratories that are into cellular or systems-level of localization of proteins, including other cellular constituents.

The general truth about this method is that it has become so terrestrial that many practitioners take for granted that an antibody put up for sale for the localization of a specific molecular target will both have sensitivity and specificity. In the present state of extensively accurate analyses of DNA by in situ hybridization, DNA chip analyses, and deep sequencing, it is often believed that immunohistochemical can analogously and accurately spot molecular targets.
What is the specific antibody?
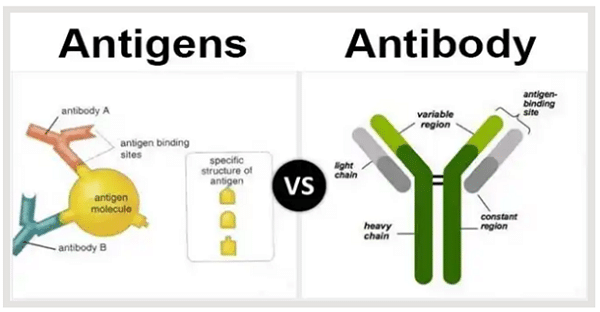
Antibodies such as CHAT antibodies can help eradicate certain foreign substances when they bind to certain antigens. When a specific pathogen such as a harmful bacterium or virus strikes the body, the antibody’s variable region binds to the harmful bacterium that enters the body. It can eventually result in a wide range of actions when this happens, such as eliminating pathogens. Antibodies that attack cancer cells, for instance, do not attack normal cells. This is a result of anybody recognizing a certain antigen. This is referred to as the “specificity” of an antibody toward that antigen. In this manner, dangerous pathogens to the body are eliminated by antibodies by specific recognition and identifying bacteria and viruses.
An antibody is a molecule with a combination property, specifically with a second molecule known as the antigen (protein). Moreover, antibody production by an animal is induced specifically by the presence of the corresponding antigen. The helps in constituting the response of the basic immune system. The recognition of antigen-antibody depends on three-dimensional (3D) protein structure or some other antigen which may be compromised by formalin-induced modification of protein conformation (“masking”) but is restored in part by AR. Antibodies are immunoglobulin molecules consisting of two basic units: a pair of light chains, either a kappa or a lambda pair, and a pair of heavy chains—gamma, alpha, mu, delta, or epsilon.
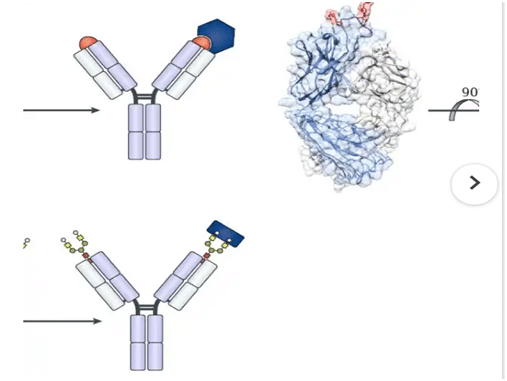
You can view Antibody specificity as either a measure of the goodness fit between the combining site of an antibody (paratope) and the corresponding antigenic determinant (epitope). Alternatively, you may view it as the ability of the antibody to criticize between antigens that look alike or the one that dissimilar antigens (Candler et al., 2006).
Principles of Antibody Action
As said earlier, acetylcholinesterase antibodies are proteins in the immune globulin family. However, they are produced by B-cell lymphocytes being part of the response of the adaptive immune system. Unusual genes are known to code immune globulin, containing a variable region that is different between B cells but remains the same for the entire life of the individual B cell (Neuberger 2008). An immune globulin variable region enables it to bind or attach to a molecular target, fitting perfectly into the site of a bid. However, these immune globulin specificity binding sites can be exquisite, recognizing an R vs. S enantiomer of the exact molecule or identifying proteins only when they pass through the phosphorylation process.
On the other hand, a variable region that binds a common molecular motif may not be difficult to bind to many targets. The truth about this is that recognizing a molecular motif may be a function of tertiary folding. There will not even be a need to be a series of consecutive amino acids in a protein.
Antibodies Against Different Portions of the Same Molecule
A related topic can be useful in generating antibodies to synthetic peptides that are not derived from the same components of the same molecule. However, there’s a possibility about this, for instance, having antibodies against a large protein target that specifically bind to the N- or the C-terminal portions of the protein. The possibility provides a useful potential for determining the specificity of the antibody, such as the MM9 antibody. When the two different antibodies stain just in the exact pattern, there are higher chances of straining the correct target.
Conclusion
Different molecular components are present in a natural immunogen responsible for exciting the response of antibodies. However, the resulting antibodies will be caused by a clonal expansion of one or more B cells. Therefore, investigators need to understand the potential antibody-specific problems for proper documentation.